If the electron has wave properties, then it ought to be possible to observe these: electrons should show the characteristics of waves such as interference and diffraction. This is clearly a difficult concept if we are used to thinking of the electron as a particle. We must, however, abandon the out of date idea that the electron, or in fact any particle, always behaves like a solid object!
The very small wavelength of electrons means that the obstacles used to diffract them must also be very small, and as with X-rays it was the atomic lattice that was eventually found to be suitable.
The diffraction of electrons was
first shown by Davisson and Germer in 1927
and it can now be observed easily in schools with the correct apparatus. A
very good example is manufactured by Teltron Ltd, in which a beam of electrons
is accelerated in an electron gun to a potential of between 3500 and 5000 V and then allowed to fall on a very thin sheet of graphite
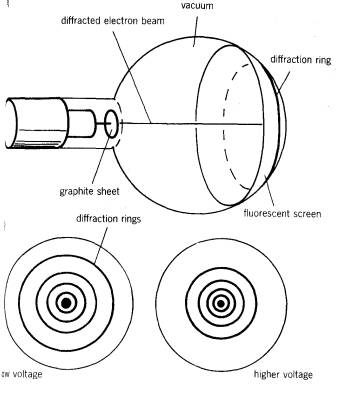
The electrons diffract from the carbon atoms and the resulting circular pattern on the screen is very good evidence for the wave nature of the electrons.
The diffraction pattern observed on the screen is a series of concentric rings, just as light is diffracted by an irregular obstacle such as dust particles round a street lamp. This is due to the irregular spacing of the carbon atoms in different layers in the graphite.
If the voltage on the anode is increased the energy of the electrons is increased, and the diameter of a given ring gets less. This is exactly similar to the observation that blue light is diffracted less strongly than red light, which arises because the wavelength of blue light is smaller than that of red and hence its energy is larger
.
Quantised orbits
The simple Rutherford model of the atom had one serious disadvantage concerning the stability of the orbits. Bohr showed that in such a model the electrons would spiral into the nucleus in about 10-10 s, due to electrostatic attraction. He therefore proposed that the angular momentum of the electron should be quantised, in line with Planck's quantum theory of radiation.
He stated that the allowed values of the angular momentum would be integral multiples of h/2p that is,
Angular momentum = nh
2p
This implied a series of discrete orbits for the electron. From the Schroedinger wave equation we can imagine the electron as existing as a wave that fits round a given orbit an integral number of times. In other words, if r is the orbit radius and l the wavelength,
2pr = n l
A very simple analogy of this is seen if we fit a circle of copper wire to a vibrator and then oscillate it as shown:
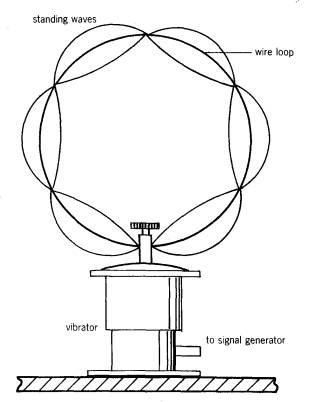
A series of resonance positions can be found where an integral number of waves fit round the orbit.
Verification of Bohr's principle
The idea of the electron's discrete or quantised orbits within an atom can now be verified. We have two proposals:
(a) that of Schroedinger, suggesting that n electron waves can be fitted around an orbit, that is
![]()
(b) that of de Broglie, who proposed that electrons have a wavelength:
![]()
Therefore
![]()
But the quantity mvr is the angular momentum of the electron, and therefore Bohr's original proposal had been confirmed, since n is an integer. Within a few years of 1923 the wave hypothesis was developed into the theory known as wave mechanics, by Schroedinger and others.
Each electron also possesses an internal angular momentum called spin, which must be conserved in nuclear and atomic processes. Spin gives rise to a magnetic moment of the electron within the atom.