9.2.5 The
discovery of photoelectricity - Link
to a cheesy web site
Metals can emit
electrons when they are supplied with sufficient energy. In thermionic emission
this energy is supplied by heating the wire with an electric current. In the
case of photoelectricity the energy is supplied by illuminating the surface of
the metal with ultra violet light. This was first discovered by Heinrich Hertz
during his work on radio waves but he did not follow up his discovery.
Further investigations showed that other metals can emit photoelectrons. Photoelectrons are identical to any other electron, the prefix Ďphotoí just indicates how the electron gained its energy. Measurements on the photoelectric effect showed that;
∑ the number of photoelectrons emitted per second from any metal is proportional to the intensity of the illumination.
∑ photoelectrons emitted from a metal have a range of kinetic energies up to a maximum value.
∑
no photoelectrons are emitted if the frequency of the incident light fall
below a certain threshold frequency irrespective of how intense the light is.
The existence of a threshold frequency presented a great puzzle to scientists of the time. According to wave theory there should not be a threshold frequency at all. Each electron should take energy from each incident light wave until it gained sufficient to leave the metal surface. With low frequency light emission should take longer to occur but it should eventually happen. It doesnít !
Einsteinís theory
was developed from the quantum theory put forward a few years earlier by Max
Planck to explain the black body radiation curves (see earlier). In 1905
Einstein extended Planckís ideas and used them to explain the existence of the
threshold frequency in the photoelectric effect.
He suggested that the
quantum of energy emitted by an atom continued to exist as a concentrated packet
of energy. So when an atom emits light it loses a Ďlumpí of energy carried
away by a wavepacket, which we call a photon. The idea of a wavepacket helps to
visualise the idea of a photon which is different to waves on water. A
wavepacket is a concentration of energy that travels away from the source in one
direction only. A source of light emits photons travelling in all directions but
each photon travels away only in one specific direction.
The energy of a
photon is proportional to its frequency and is given by
![]()
where h = Planckís constant
f = frequency
If one considers a
stream of light directed at a metal surface. Any free electron near the surface
of the metal could be struck by a photon and thus gain kinetic energy. If
sufficient energy is gained the electron may leave the metal surface. The energy
gained must be equal to the whole energy of the photon (hf).
To escape from the
metal the electron must do work. The electron can only escape if it gains
sufficient energy from an encounter with a single photon. Thus the photon energy
must be greater than the work needed to escape from the metal surface and thus
the maximum KE of the photoelectron will be
![]() or
or
![]()
where
W = work function of the metal (the work necessary to remove an electron
from the metal)
This equation is
known as Einsteinís photoelectric equation.
1.
Why do the emitted photoelectrons have a range of KEís even if
monochromatic light is used ?
2.
Show that the threshold frequency is given by
![]()
3.
What would happen to the photoelectric emission if the metal is at
a)
a positive potential
b)
a negative potential
Einsteinís
theory was proven by Millikanís experiments in 1916. If a positive voltage is
applied to the metal then it will stop photoelectric emission at a particular
frequency when the voltage reaches a value known as the stopping voltage Vs.
Extra work equal to eVs has to be done to remove the electron from
the metal and thus photoelectric emission will stop when
![]()
Einstein
predicted that a graph of Vs against frequency would have the same
gradient irrespective of the material used.
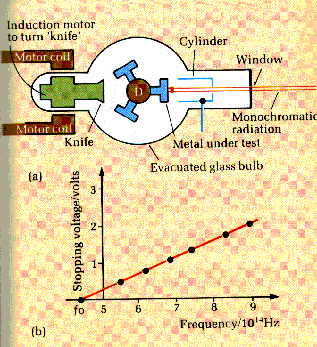
Millikan
devised a vacuum tube in which clean metal surfaces could be cut. He used a drum
with three different metals. The drum could be rotated and a freshly prepared
surface could be obtained using a knife which could also be rotated using an
induction motor arrangement. Light of a selected frequency could be obtained
from a spectrometer and shone onto the prepared metal surface. A metal cylinder
collected any photoelectrons released from the metal. An electroscope was used
to show the charge collected by the cylinder. Millikan then increased the
potential of the metal surface until the electroscope showed that no
photoelectrons were being emitted. This potential was then equal to the stopping
potential for that frequency of light.
Millikan
made a series of measurements for each metal over a range of different
frequencies. The results he obtained fitted Einsteinís predictions exactly and
gave a value for h of 6.6 x 10-34 Js.
The
main features of the graph are
∑
the gradient for any metal is always
equal to h/e
∑
the intercept on the f axis is equal to
f0, the threshold frequency of the metal, hence the work function of
the metal may be calculated.