Towards absolute zero
Absolute zero is the lowest possible temperature. Physicists have achieved temperatures lower than 0.001 K. Strange effects have been observed at very low temperatures. For example, metals become superconductors, losing all trace of electrical resistance. Helium‑4 liquefies at 4 K and becomes a superfluid below 2 K, capable of creeping out of an open container. Such discoveries became possible as a result of being able to reach very low temperatures.
Cooling processes
Cooling by evaporation If you have ever been inoculated, you may remember that the skin is dabbed with a volatile liquid such as ether to numb the pain. The liquid evaporates from the skin, cooling the skin sufficiently to make it insensitive. The more energetic molecules of the liquid evaporate, leaving less energetic molecules in the liquid which is therefore cooler.
Refrigerators in series A refrigerator contains a liquid that absorbs energy and vaporizes in one part of the refrigerator and then condenses and releases energy in a different part. The lowest temperature possible in a refrigerator is determined by the boiling point of the liquid under reduced pressure.
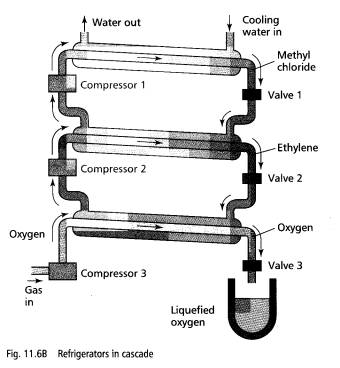
Oxygen was first liquefied in 1877 by using a series of 'refrigerating machines', each with a different refrigerant liquid. The first machine was used to cool the condenser of the second machine which was used to cool the condenser of a third machine. Using ethylene as the second refrigerant, the temperature of the third condenser was reduced to 103 K which is below the critical temperature of oxygen. Hence, oxygen could be liquefied by compression in the third condenser. Nitrogen can also be liquefied by this process.
Cooling by adiabatic expansion
If a gas expands, pushing back the atmosphere as it does so,
it will cool provided there is no heat transfer between the gas and its
surroundings. This happens because the gas uses some of its own internal energy
to expand. This is an example of an adiabatic
expansion.
Suppose the air in a cycle pump is compressed, allowed to gain thermal equilibrium with the surroundings, then released suddenly. On release, it expands and pushes back the atmosphere. Since the release is rapid, there is no heat transfer during the release process so the work done by the gas to expand comes from its own internal energy. Hence the gas cools. Oxygen, which has a boiling point of 90 K, and nitrogen, which has a boiling point of 77 K, can both be liquefied by this process.
The Joule-Kelvin effect None of the processes described above can be used to liquefy hydrogen, which has a boiling point of 33 K. The Joule-Kelvin effect is used for this. It can achieve temperatures of less than 1 K
The effect was discovered in 1853 by Joule and Kelvin who were investigating the temperature change of different gases forced through a narrow outlet from constant high pressure to a constant low pressure.
Gas at constant high pressure
![]()
Inlet
Outlet
Gas
at constant
low pressure
Their results showed that the temperature change depends on the initial temperature, the initial pressure and the final pressure. The effect is due to intermolecular forces. For the same initial temperature and pressure, the final temperature varies according to the selected value of the final pressure as shown by the curve XY in Fig. 11.6C. To achieve cooling, the final pressure must be less than the pressure at the highest point on this curve, otherwise a heating effect is always achieved.
Typical measurements on a gas for different initial
conditions give results which may be plotted as a set of temperature v pressure
curves. The locus of the highest points is referred to as the inversion curve.
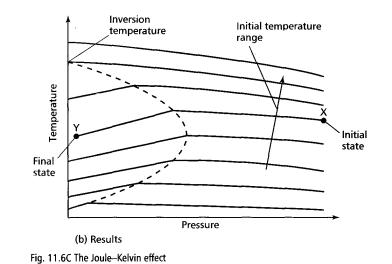
The inversion temperature is the temperature above which no cooling effect is possible. This is the point on the temperature axis at which the inversion curve cuts the temperature axis. If the temperature of a gas is above its inversion temperature, the Joule-Kelvin effect cannot produce a cooling effect, regardless of the initial and final pressures.
The inversion temperature for oxygen and nitrogen is above 600 K; hence these gases can be cooled using this effect without pre-cooling. However, the inversion temperatures of hydrogen and helium are 202 K and 33 K respectively so these gases need to be pre-cooled to produce the effect.
An outline of a practical system used to liquefy hydrogen is shown here:
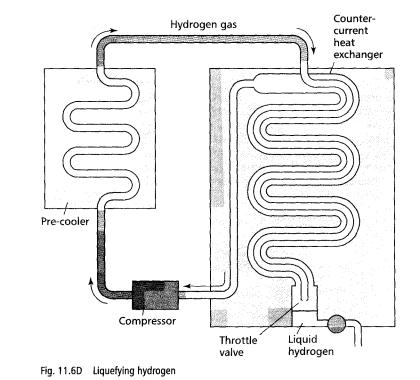
1 The gas is compressed then pre-cooled by passing it through a metal pipe in liquid air.
2 The gas is then forced through the inner tube of a double-walled pipe throttle valve. This lets the gas through a narrow outlet into a sealed container at low pressure, causing it to cool in the process.
The gas is now cooler than
before and passes through the outer wall of the double-walled pipe, cooling the
incoming gas even more, before returning to the compressor. The double walled
pipe is called a countercurrent heat
exchanger.
4 The incoming gas has been cooled even more by the countercurrent heat exchanger so when it passes through the throttle valve, its temperature is lowered even more.
5 Eventually the incoming gas is so cold that it liquefies after passing through the throttle valve. The liquefied gas then collects in the sealed container where it can be tapped off into a suitable flask.
Pre-cooling can also be achieved by expanding the gas adiabatically. This is more effective in practice than pre-cooling using liquid air or other cold liquids.