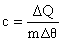|
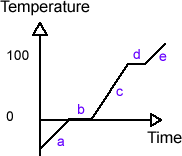
In GCSE, you probably did an experiment where you took some ice, heated it steadily with an electric heater and measured the temperature as time passed. You should have got these results:
Even though you heated constantly, the temperature only rose sometimes. So where was the heat going when the temperature wasn’t rising? Answer – it was being used to change the state of the water. Remember in the energy topic we said that the energy of an atom was made up of two components – Ek and Ep. Well: The Ek component relates to the temperature of the substance The Ep component relates to the state of the substance. So during times of increase of temperature (e.g. a, c and d from the graph) the heat energy supplied is going into the Ek component of the internal energy. And during changes of state (e.g. b and d) it’s going to Ep. Because Ek isn’t changing during these times, the temperature remains the same.
|
|
|
If you look back at the graph, the straight line sections suggest that to change the temperature of water by 1oC takes a certain amount of energy whether the rise is from 1 to 2oC or from 90 to 91oC. This is true! The amount of energy required is called the Specific Heat Capacity. The definition of Specific Heat Capacity is: “The amount of energy required to raise the temperature of 1 kg (a unit mass) of the substance by 1oC (a unit temperature rise)” Symbol: c Unit: Jkg-1K-1
where
m is the mass of the substance you are heating (or cooling)
Note – the value of ‘c’ for ice isn’t the same as that for water or for steam. However, the value of ‘c’ is the same if you are cooling rather than heating the substance. In other words you get as much heat back out of thee substance when you cool it as you put in when you heated it. |
|
|
It takes a certain amount of energy to change the state of 1kg of water from solid to liquid. This amount of energy is called the Specific Latent Heat, lf, of water The definition:
where:
There is no temperature term involved in this equation as it all takes place at the same temperature. Note that there are two occasions when you change state and both of these require different amounts of energy (as different things are happening to the atoms during the state changes). So there are two symbols. lf – latent heat of fusion – solid to liquid and back. lv – latent heat of vaporisation – liquid to gas and back. You can actually have ls – latent heat of sublimation – solid to gas and back. But you don’t often come across that. So back to the graph.
a, c and e – Heat energy goes to change the Ek component. Specific heat capacity equations apply. b and d – Heat energy goes to change the Ep component. Specific latent heat equations apply. |
|