|
Thermometric properties |
|
|
To measure the temperature of an object you first need to find something that varies with temperature. This is called a Thermometric property. For example, the volume of mercury, the resistance of a piece of wire and the pressure of a gas in a fixed volume. |
|
|
The Centigrade scale |
|
|
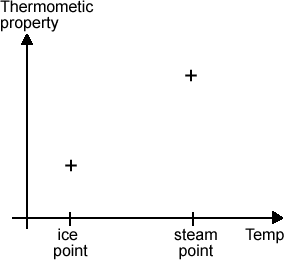
To measure the temperature of the object, put the thermometric property in thermal contact with the object so that they can both become the same temperature, and then measure the change to the thermometric property and this gives you a way of measuring the temperature change. Obviously, you need to find out how the thermometric property varies with temperature first so that you can interpret any change. To do this, measure the value of the thermometric property at two known temperatures e.g. the freezing point of water (ice point) and the boiling point of water (the steam point). These two points are called fixed points. Your results will look like this.
The simplest thing to do now is to draw a straight line linking the two points together, divide the line into 100 equal steps (and call the steps degrees) and that’s it! This is called a centigrade scale. |
|
|
Absolute or Thermodynamic Temperature Scale |
|
| Scientists have
developed an ideal, perfect scale with an absolutely
straight-line relationship between temperature and the
thermometric property. This scale is called the absolute
scale or the thermodynamic scale of temperature.
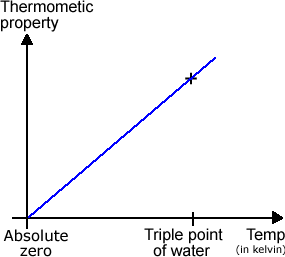
The problem is that it is a theoretical scale (although some constant volume gas thermometers can get close to its perfect behaviour.) But that doesn’t mean that it is any less useful. It is based on absolute zero. (It has never been reached in practice although we’ve come within a few millionths of a degree). Absolute zero is the coldest temperature at which all molecular motion is zero and all the substance is perfectly ordered. Absolute zero is one of the two fixed points for the Absolute scale of temperature. The other is the triple point of water – a point at very low pressure at which water can exist as a solid, liquid and gas all at the same time – hence the name ‘triple point’. It is in fact 273.16 degrees above the absolute zero point. So the absolute scale temperature graph looks like this.
The units for the absolute scale of temperature are kelvin, K. (Note – they are not degrees kelvin.) On this scale, the ice point is 273.15 K and the steam point is 373.15 K. Note – Kelvin carefully chose the size of the steps on his absolute temperature scale to give exactly 100 steps between the ice and steam points to avoid confusion. So the difference between the ice and steam points is 100oC or 100 K. Note – the triple point of water is 273.16K, just a little above the ice point. Whenever you do calculations using temperature (usually in gas equations) you need to use the absolute or Kelvin values of temperature. |
|
|
The Celsius Scale |
|
|
The absolute scale would never have caught on as a temperature scale for everyday use. Can you imagine two old ladies chatting in a bus queue on a cold day. One says “Blimey, it’s cold!” and the other replies “Yes – it must be 273.15 kelvin!” So someone thoughtfully developed the Celsius scale that is exactly the same as the absolute scale except that the numbers are in a different place. The ice point has been named zero Celsius so the absolute zero temperature is –273.15oC and the steam point is 100oC. A quick equation allows you to convert easily from one to the other. Temperature in Celsius = temperature in Kelvin – 273.15 Remember – in calculations, always use the kelvin value. |
|