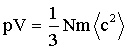|
So far our discussion has been about the property and behaviour of a gas on a big (macro) scale i.e. pressure and volume, which involve billions of atoms or molecules. Kinetic theory takes the view that if you really want to understand what’s going on you need to look at how individual atoms are behaving. Ideal gases and Internal Energy Normally we consider the internal energy of a substance to be made up of two components - Ek and Ep. The Ek is the vibration, rotation or translation of the atoms. The Ep is due to the interaction of one atom and its neighbours. The impact of these two components on the temperature and state of the substance they belong to is discussed at the start of the ‘Thermal Properties’ section. Once again as with our earlier gas work, we need to imagine an Ideal Gas that behaves in a perfect way. We’ve already stated that an Ideal Gas is one that perfectly obeys the Gas Laws. Here’s something else about Ideal Gases. An Ideal Gas is one in which all its internal energy is in Ek form. i.e. it has no Ep. How can you justify this? Well Ep is as a result of one atom exerting a force on its neighbours. In ideal gases, each atom acts independently. It is unaware of the fact that any other atoms exist (except during collisions - but they only last for an instant!) |
|
Brownian Motion |
|
|
About 150 years ago a botanist, Robert Brown, observed pollen grains moving in a random way under his microscope. You can see the same effect with smoke today. We call it Brownian Motion. Brown thought the movement of the grains may be explained by the fact that the pollen was alive! But in fact it was some years before it was explained that the movement was due to collisions between the pollen and millions of smaller (and therefore invisible) gas atoms. The pollen was small enough to be knocked sideways when more air particles collided with one side of it than with the other. This was evidence of the continuous movement of gas atoms. Gas atoms have Ek. |
|
Kinetic Theory Assumptions |
|
|
Kinetic Theory then developed to explain this movement. The following assumptions are made to help the theory:- 1) There are a very large number of particles (molecules or atoms) involved. This means we can apply statistics to our solutions. 2) The particles are involved in perfectly elastic collisions with their containers and each other. So no Ek is lost. 3) The length of time involved in a collision is
negligible compared to the time between collisions (i.e. we can
ignore the moments when the potential energy component of the
internal energy is not zero).
where p = pressure V = volume N = No of atoms/molecules m = mass of one atom/molecule
and
where
because
You are required to learn how to derive these from scratch.
|
|
Root mean square speed, rms speed |
|
|
The mean square speed, This is not the same as the mean speed or average speed of
the molecules but it is easily measurable (using
|
|
Boltzmann constant and Ek |
|
|
Remember: pV = nRT where n = number of moles of gas and R = universal molar gas constant. Boltzmann decided it would be useful if we know what the gas constant was per molecule, not per mole. He came up with a new constant - named the Boltzmann constant, of course - which was:
where k = the Boltzmann constant R = the universal molar gas constant NA = Avogadro’s number, the number of particles in one mole. Then it was possible to rewrite pV = nRT as pV = NkT where N = no of atoms or molecules involved. If you combine
and pV = NkT to give
or, in words The average Ek of the particles = a constant x temperature (in kelvin). This confirms what we’ve mentioned a number of times in this section, that the temperature of a gas depends only on the Ek of the molecules that make it up. |
|