Gas law questions - with answers
Question 2.1.
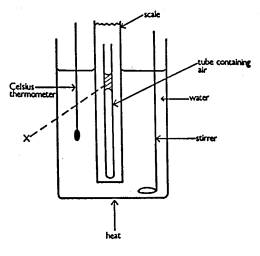
The diagram shows a fixed mass of dry gas contained
in a flask. The pressure exerted by the gas can be measured by the mercury
manometer connected to it by a narrow tube - as shown in the diagram
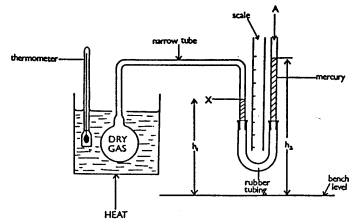
The flask of gas is immersed in a beaker of water,
which can be heated. A thermometer records the temperature of the water. The
volume of the gas is kept constant.
(a)
Explain how adjustments are made to keep the volume of the gas constant.
(b)
What pressure is represented by the symbol A in the diagram?
(c)
Consider the diagram; does the gas exert a pressure equal to A, less than A or
greater than A? Explain your answer.
A student performs an experiment to investigate how
the pressure the gas exerts varies with its temperature when its volume is
kept constant. The student obtains the following results.
|
Pressure
A = 73.7 cm of Hg |
(Hg
= |
mercury) |
|
Temperature
(oC) |
h1
(cm) |
h2
(cm) |
|
24 |
28 |
38.8 |
|
40.5 |
28 |
43.2 |
|
63 |
28 |
49.9 |
|
76.5 |
28 |
54 |
|
97 |
28 |
59.4 |
(d)
Draw up a table showing the variation of temperature (oC)
with total
pressure exerted by the gas.
(e)
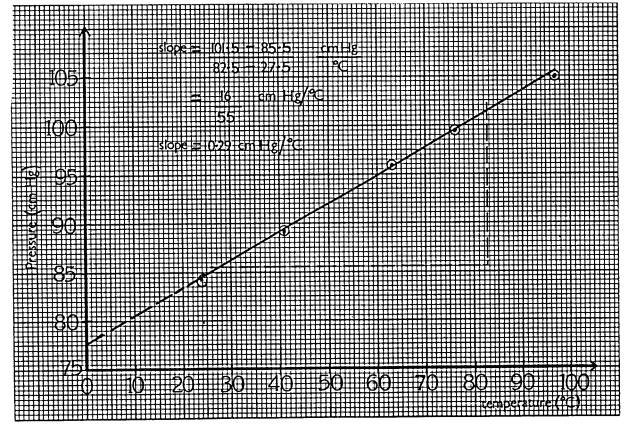
Draw a graph of pressure (y-axis) against temperature (x-axis)
(f)
Find the slope of your graph.
(g)
Use the graph to find the pressure exerted by the gas at 0oC
(h)
At what temperature would you expect the pressure to be zero? Explain the
significance of this temperature
Answer to
Question 2.1.
(a) By
raising or lowering the right hand side of the mercury manometer, the level on
the left-hand side is brought back to X - the constant volume mark.
(b) A represents the atmospheric pressure.
(c) The gas exerts a pressure greater than the atmospheric
pressure. We see this because it pushes the mercury back against the
atmospheric pressure - the mercury height on the right hand side being greater
than on the left-hand side of the manometer.
(d)
|
Temperature
(oC) |
*Total
pressure exerted by gas (cm Hg) |
|
24 |
84.5 |
|
40.5 |
88.9 |
|
63 |
95.6 |
|
76.5 |
99.7 |
|
97 |
105.1 |
*{Total gas pressure = A + (h2 - h1)] cm of Hg)
(e) & (f )
(g)
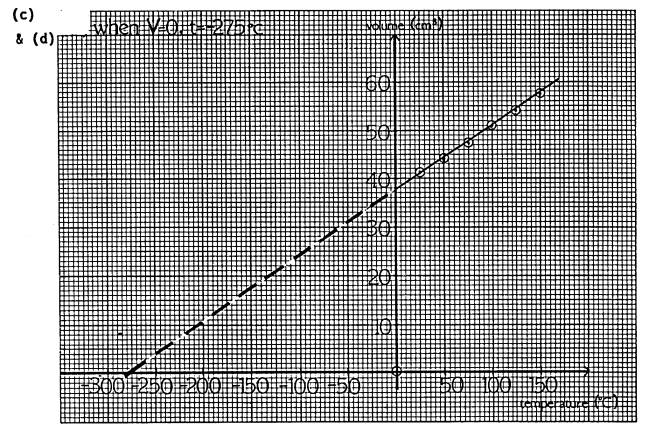
When t = 0oC, p = 77.8 cm Hg
(h) p = 0 when t = -2730C. This value is taken as zero on the absolute(Kelvin) scale of temperature - it is absolute zero.
SECTION
2 : STRUCTURE OF MATTER
Question
2.2.
(a) Name
and state the law which describes how the pressure of a fixed mass of gas
varies with volume if its temperature is kept constant. Express this law as a
mathematical formula.
(b) Describe
the graph you would plot in order to verify this law. Explain your answer.
(c) A diving bell contains 17.5m3 of air at the
surface of the water. It is lowered to a depth where the pressure is 5
atmospheres. Calculate the volume of air now in the diving bell - assume the
temperature of the water has remained constant.
(d) Explain, in simple language, how the kinetic theory
accounts for the behaviour described by this law.
Answer to Question 2.2.
(a) The law is called Boyle's law. The law says that for a fixed mass of gas kept at constant temperature the pressure the gas exerts is inversely proportional to its volume.
P a 1/V
so p = k x 1/V
p
= the pressure
k = constant of proportionality
V
= the volume
:.
pV = k, a constant
(b) With a series of values of p and corresponding values V,
the graph to plot to verify Boyle's law is one that has p (or 1/V ) on the y
axis against 1/V (or p) on the x-axis.
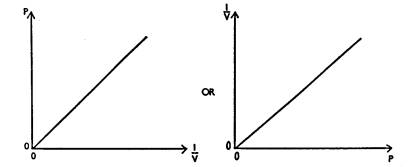
Since the graph is a straight line graph
passing through the origin, we can say:
P a 1/V
OR 1/V
a P
c) Given
V1 = 17.5m3
V2 = ?
P1 = 1 atmosphere
P2 = 5 atmospheres
Boyle's
law applies so p = k 1/V
:.
pV = k, a constant
SO
P1V1
= P2V2
:.
V2 = P1V1
P2
= 1 x 17.5
m3
5
V2 = 3.5 m3