Question
2.4.
(a)
Explain
the meaning of the terms mass number and atomic number. Use your
explanation to explain what isotopes are.
(b) Isotopes
cannot be separated by chemical means but can be separated using physical
techniques usually magnetic fields and electric fields. Explain why this is
so.
(c) Explain
and describe the effect which the alpha particle decay has on the parent
atom's mass and atomic number. Complete the following equation by including
mass and atomic numbers for a and Rn.
226
Ra à
a + Rn
88
Ra
- radium
Rn - radon
a - the alpha particle
(d) Similarly
explain and describe the effect which the beta particle decay has on the
parent atom's mass and atomic number. Complete the following equation by
including mass and atomic numbers for b and N
14 C
à
b
+ N
6
C
- carbon
b -
beta
N - nitrogen
(f) A
radioactive isotope has a half-life time period of 10 seconds. What fraction
of it remains undecayed after a time of 40 seconds?
Answer
to Question 2.4.
(a) Mass
number A - the total number of protons and neutrons in the
nucleus of an atom.
Atomic number Z - the number of protons
in the nucleus of an atom.
Isotopes
are atoms
of elements that have the same atomic numbers but different mass numbers as
they do not have the same number of neutrons in their nuclei.
(b) The
chemical behaviour of an element is determined by its electron structure. In a
neutral atom, the number of protons will equal the number of electrons. Since
isotopes of elements have the same number of protons (same atomic numbers),
they appear to be chemically the same.
(c) An alpha particle consists of two protons and two neutrons - so it has mass number of four and an atomic number of two. This means that the mass number of the parent atom decreases by four and the atomic number decreases by two.
226
Ra à
4 a +
222 N
88
2
86
(d) Beta
particles are fast moving electrons that leave the atom when a neutron decays to
an electron, the beta particle, and proton, which stays in the nucleus. The
result is that, in beta decay, the mass number remains unchanged and the atomic
number increases by one
14
C
à
0 b +
14 N
6
-1
7
(e) The
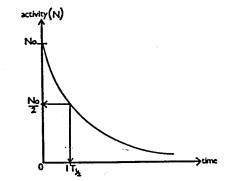
half-life time period is the time taken for half the nuclei in a sample of
radioactive material to decay away, leaving the remaining half left undecayed.
Activity
is a measure of the number of undecayed atoms (N)

(f)
1/16 of the
original sample is left undecayed
Question
2.5.
If uranium-235 absorbs a
neutron then it forms the isotope uranium-236. The reaction is described by the
following equation
235
U +
1 U -->
236 U
92
0
92
(a) Uranium-236
is unstable; describe what happens to it.
(b) Explain what is meant by the term chain reaction.
(c) What is the source of the energy released in a nuclear
reaction?
(d) Uranium-235
is more likely to absorb a neutron if the neutron is slow moving. Explain how
such neutrons are produced in a reactor.
(e) Explain how the nuclear reaction is controlled in a
reactor.
(f) Name
the alternative nuclear reaction that could in the future provide a source of
energy.
(g)
Briefly explain the principles of this
reaction - you
do not need to name the atoms involved
(h)
Where does this reaction naturally take place?
Answer to
Question 2.5.
(a)
The unstable U-236 nucleus breaks into fragments
and releases two or more neutrons
(b) The
neutrons released in the break up of the U-236 nucleus can be absorbed by the U-235
nuclei which then become unstable U-236 nuclei which break up and repeat the
process - with more and more neutrons being produced and more nuclei become
unstable.
(c) The
source of energy produced is the difference in mass; the mass of U-236 does not
equal the mass of the fission fragments plus the released neutrons. This mass is
converted into energy.
(d) Neutrons
are slowed down using a material known as the moderator placed in the
rector core.
(e) The
reaction is controlled by placing a material known as the control material
- in the form of rods - that absorb neutrons and so slow down or stop the chain
reaction
(f) The alternative process is fusion
(g) In
fusion, two light nuclei bind together to form a heavier nucleus. To bind, the
nuclei must be very fast moving which requires the material used to be at a very
high temperature.
(h) These reactions occur in the stars - such as the sun.
Question
2.6.
(a) Describe the nature of alpha particles and beta
particles.
(b) Describe
how alpha particles and beta particles lose their energy when they travel
through air.
(c) In
air, alpha particles travel for about five centimetres before losing their
energy whereas beta particles can travel for about one hundred centimeters
before doing so. Explain why this is so.
Answer
to Question 2.6.
(a) Alpha
particles are helium nuclei so they have two protons and two neutrons and a
positive charge of +2e where e is the electron charge.
Beta particles are fast moving electrons so they have very little mass and a
negative charge of -e.
(b) Alpha
particles and beta particles both lose their energy by a process of ionisation.
Each atom ionised by such a particle costs the particle a certain amount of
energy, so the number of atoms an alpha particle or a beta particle can ionise
depends upon the energy, which the particles have.
(c) Alpha
particles are massive compared with the electrons surrounding the atoms of air.
They also have double the charge and, as it is a positive charge, it strongly
attracts the electrons. The result is that the alpha particles cause a lot of
ionization and quickly lose their energy - so they only travel for a short
distance through air.
Beta particles are very, very, small in
comparison and carry half as much charge as the alpha particles. The
result is that they cause much less ionization, so they travel further before
they lose all their energy.