9Fa
1 The gas in the air that reacts with metals most easily is:
A argon. B carbon dioxide.
C nitrogen. D oxygen.
2 A metal that does not react with the air is:
A calcium. B gold.
C iron. D magnesium.
3 The gas which is given off when metals react with water is:
A carbon dioxide. B hydrogen.
C oxygen. D nitrogen.
4 Sodium reacts with water to form:
A sodium chloride.
B sodium oxide.
C sodium hydroxide.
D sodium carbonate.
9Fb
1 The gas which is given off when metals react with acids is:
A carbon dioxide. B hydrogen.
C oxygen. D nitrogen.
2 When magnesium reacts with sulphuric acid, it forms:
A magnesium oxide.
B magnesium chloride.
C magnesium sulphate.
D magnesium carbonate.
3 Look at these diagrams, and then choose
the correct order of reactivity
for metals X, Y and Z (the most reactive metal
should be first).
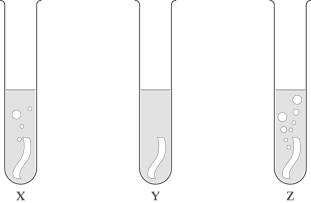
A Z, X, Y B Y, Z, X
C X, Y, Z D Z, Y, X
4 Choose the correct statement to complete this word equation:
zinc + sulphuric acid ®
A zinc + sulphur + acid
B zinc hydrate + sulphur
C zinc sulphate + hydrogen
D zinc hydroxide + hydrogen
9Fc
1 Iron is more reactive than copper. If a
piece of iron is put into copper sulphate solution,
what would you see?
A Brown copper would appear on the iron.
B Blue copper would appear on the iron.
C Nothing would happen.
D the iron would dissolve completely.
2 Zinc is more reactive than tin. It will
displace tin from a compound.
Choose the correct statement to complete this
word equation:
zinc + tin sulphate ®
A zinc + tin sulphate
B zinc + zinc sulphate
C tin + zinc sulphate
D tin + zinc chloride
3 Copper is more reactive than silver. Iron is more reactive than copper. This means that:
A copper will react with iron nitrate solution.
B silver will react with iron nitrate solution.
C iron will react with silver nitrate solution.
D silver will react with copper nitrate solution.
4 Zinc reacts with copper sulphate solution and silver nitrate solution. This means that zinc:
A is more reactive than copper and silver.
B is less reactive than copper and silver.
C is less reactive than copper but more reactive than silver.
D is less reactive than silver but more reactive than copper.
9Fd
1 Which of these materials is used to make most car bodies?
A plastic
B aluminium
C copper
D steel
2 Some window frames are made of aluminium. It is a good metal to use because:
A it conducts electricity.
B it conducts heat.
C it does not melt easily.
D it does not react very quickly with air or water.
3 Magnesium would react more slowly with acid if you:
A added more acid.
B added more water.
C heated the acid.
D used a more concentrated acid.
4 Which of these statements is true?
A Many reactive metals have been known since ancient times.
B Magnesium was discovered before iron.
C Reactive metals can be extracted by heating their compounds with charcoal.
D Most reactive metals were discovered after the invention of the electric battery.