8F Quick Quiz
1 An atom is:
A the smallest particle that can exist.
B a group of particles that are all the same.
C the smallest particle of a compound that exists under normal conditions.
D the smallest particle of an element that exists under normal conditions.
2 A molecule is:
A the smallest particle that can exist.
B the smallest particle of an element that can exist.
C two or more atoms chemically joined together.
D the same as an atom.
3 The number of types of atom in a compound is:
A only one.
B one or two.
C two or more.
D three or more.
4 How many different elements are there in the compound with the formula NH3?
A one
B two
C three
D four
8Fb
1 Which of these things does not show that a reaction has occurred?
A The mixture gets hotter.
B The substance changes from a liquid to a solid.
C The mixture changes colour.
D Bubbles of gas form in the mixture.
2 The substances that you get at the end of a chemical reaction are called:
A reactants.
B chemicals.
C products.
D gases.
3 Which of these changes is a chemical reaction?
A Melting some margarine in a pan.
B Boiling a kettle of water.
C Frying an egg.
D Cooling a drink by putting an ice cube in it.
4 Complete this word equation:
sodium carbonate + copper sulphate ® sodium sulphate + ____________
A copper sulphate
B copper oxide
C sodium carbonate
D copper carbonate
8Fc
1 A mixture is:
A just one substance, like pure salt.
B two or more things mixed together, like nuts and raisins.
C two things chemically joined together, like flour and sugar in a baked cake.
D a lot of the same thing put together.
2 Which of these is a mixture?
A oxygen
B air
C sugar
D common salt
3 Pure water is:
A water which is clean enough to drink.
B water which runs off the hills.
C rain.
D water with nothing dissolved in it.
4 The taste of a bottle of mineral water depends on:
A what the bottle is made from.
B which chemicals dissolved in the water as it passed over the rocks.
C the colour of the bottle.
D whether you drink it from the bottle or a glass.
8Fd
1 Orange squash is a mixture because:
A it is safe to drink.
B it always contains the same proportions of each ingredient.
C it has a number of different ingredients which can be in different amounts.
D it is a single pure substance which cannot be split up.
2 Which list contains the three gases which make up over 99.9% of the air?
A hydrogen, oxygen, carbon dioxide
B argon, nitrogen, hydrogen
C argon, nitrogen, oxygen
D argon, oxygen, carbon dioxide
3 How can the different gases in the air be separated?
A fractional distillation
B crystallisation
C filtration
D chromatography
4 How could you separate a mixture of iron and sulphur?
A filtering
B using a sieve
C evaporation
D using a magnet
8Fe
1 The melting point of a pure substance is:
A the same as the freezing point.
B the same as the boiling point.
C lower than the freezing point.
D higher than the boiling point.
2 Which row in the table shows the correct freezing and boiling points for water?
|
|
Freezing point |
Boiling point |
|
A |
32 ºC |
100 ºC |
|
B |
32 ºF |
100 ºF |
|
C |
0 ºF |
100 ºF |
|
D |
0 ºC |
100 ºC |
3 If you add impurities to a substance, what happens to the melting and boiling points?
|
|
Melting point |
Boiling point |
|
A |
stays the same |
changes |
|
B |
changes |
stays the same |
|
C |
changes |
changes |
|
D |
stays the same |
stays the same |
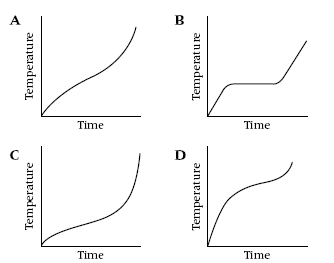
4
These graphs show what happens to the temperature when four different
substances are heated. Which graph shows a pure substance?
