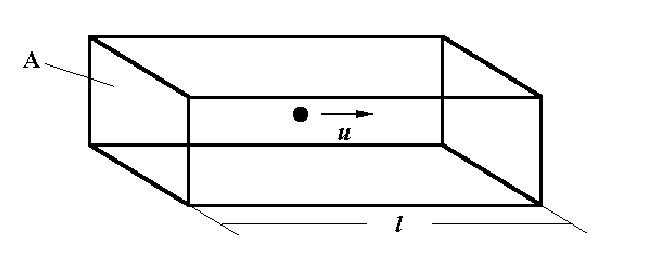
The Kinetic Theory of Gases and the Ideal Gas Equation
Consider a rectangular box length l, area of ends A, with a single molecule travelling left and then right the length of the box because of a collision with the end wall.

The time between collisions with the left wall is the distance of travel between wall A and the other wall divided by the speed of the particle in the x-direction, u.
t = 2 L
- (equation 1)
u
According to Newton, force is the rate of change of the momentum
F =
D (m u)
Dt
The momentum change upon collision is the momentum after the collision minus the momentum before the collision To hit the left wall the initial velocity must have been -u, so:
change in momentum, D (m u) = m u - (-m u) = 2m u
The average force on the left end wall is the rate of change of momentum
F
= 2m u
Dt
Combine equation (1) into the above equation and we get:
F
= 2m u
(2 L /u)
The 2's cancel and the formula reduces to:
F
= m u2
L
The pressure, p, exerted by this single molecule constrained to move in one horizontal direction (one dimension) is the average force per unit area
p = F = m u2
= m u2
A L .
A V
where V = A . L
is the volume of the rectangular box.
| Now consider N gas molecules in the
box
p = Nm u2 But they could be moving moving with velocities in ALL directions - not just horizontally. They could be moving in the: |
|
|
Using the rule for adding vectors at right angles to each other - we have to use Pythagoras to add the three velocities. (Square all the velocities and add them)
"mean square speed" of the gas molecules: c2 = ux2 + uy2 + uz2
but on average only a third of all molecules will be
moving in any given direction,
so ux2 = uy2
= uz2
and so c2 = 3 ux2 OR ux2 = 1/3 c2
If the molecules are free to move in three dimensions, they will hit walls in one of the three dimensions one third as often. The pressure then of a gas sample of N molecules in 3-D is
p
= 1 Nmc2
3 V
pV
= 1
Nmc2
3
where p = gas pressure
V = gas volume
N = number of
molecules
m = mass of each
molecule
c2
= mean square speed of the gas molecules
You need to learn this derivation by heart
Good luck to you !